Hiring an AI Developer? Here’s...
July 10, 2025

The healthcare industry is on the verge of a transformational revolution, energized by new incorporations of technology and medical practice. One of the most remarkable new ideas is called Software as a Medical Device, or SaMD for short.
This is using extraordinary computer programs to assist doctors in focusing on patients, figuring out what’s up with them, and settling on the best course without the requirement for traditional medical equipment. This critical shift towards the utilization of advanced devices in healthcare is empowering since it can work on medicine’s precision and personalization. Nevertheless, it additionally suggests that doctors, those responsible for medical care guidelines, and patients will deal with new issues and chances to learn.
As we get familiar with SaMD, we will find out how it might further develop healthcare, assist individuals with recuperating quicker, and introduce another period of tech-savvy health solutions.
Software as a Medical Device, or SaMD, is a new idea where a computer program can finish the work of a medical device. This thought comes from a gathering called the International Medical Device Regulators Forum. What’s extraordinary about SaMD is that it needn’t bother with any actual contraptions to work.
Thus, rather than utilizing a machine, you can utilize this software to keep an eye on health, sort out what’s up with somebody, or assist them with improving. SaMD is turning out to be more famous and deals with a wide range of tech stuff, regular computers, and even over the internet.
Already, this sort of software could have been known by a few names, for example, “standalone software,” “medical device software,” or “health software,” which once in a while prompts disarray with other non-medical programming types. SaMD addresses a critical shift towards utilizing digital solutions in medical services, offering a large number of utilizations to work on quiet consideration and therapy results.
All over the planet, the principles for Software as a Medical Device (SaMD) can be different depending upon the country. This is because better places have their associations and rules to ensure these digital health tools are safe, work well, and keep people’s information private.
This large number of rules and associations is significant in light of the fact that they help ensure that the SaMDs can be utilized securely in medical services, helping individuals without creating any issues. Every country’s approach to taking care of it shows they truly care about guarding patients while likewise staying aware of new tech in healthcare.
Also Read: Healthcare Digital Transformation: Telemedicine Technology
The SaMD (Software as a Medical Device) Programming Model resembles a recipe book for designers making software that behaves like a medical tool. It guides them through the entire cycle, from the planning phase to when the software is going, and in any event, when it needs fixing or refreshing.
This model spotlights ensuring the software is protected, works well, and keeps the guidelines set for health applications. It incorporates strict steps to manage risks, check quality, review how well it works medically, and protect any personal health information it handles.
Developers likewise need to ensure the product is not difficult to utilize and can function admirably with other medical equipment or systems. Following this model assists developers with making dependable, powerful, and secure clinical programming that can be utilized in clinics and centers to assist with taking care of patients better, make judgments, and plan treatments.
It’s particularly significant because it assists them with exploring the confounded guidelines of medical devices and meeting the high safety and performance standards expected by professionals and controllers from one side of the planet to the other.
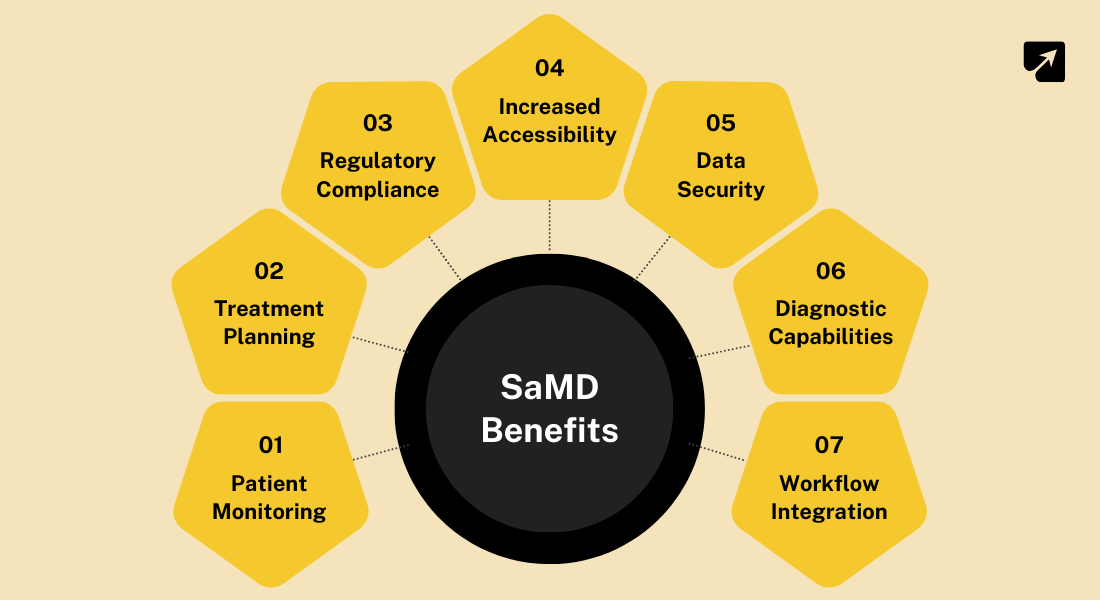
Checkout: AI-Powered EHR Software Development
The Software Development Process for Software as a Medical Device (SaMD) involves several key steps to ensure the software is safe, effective, and meets regulatory standards.
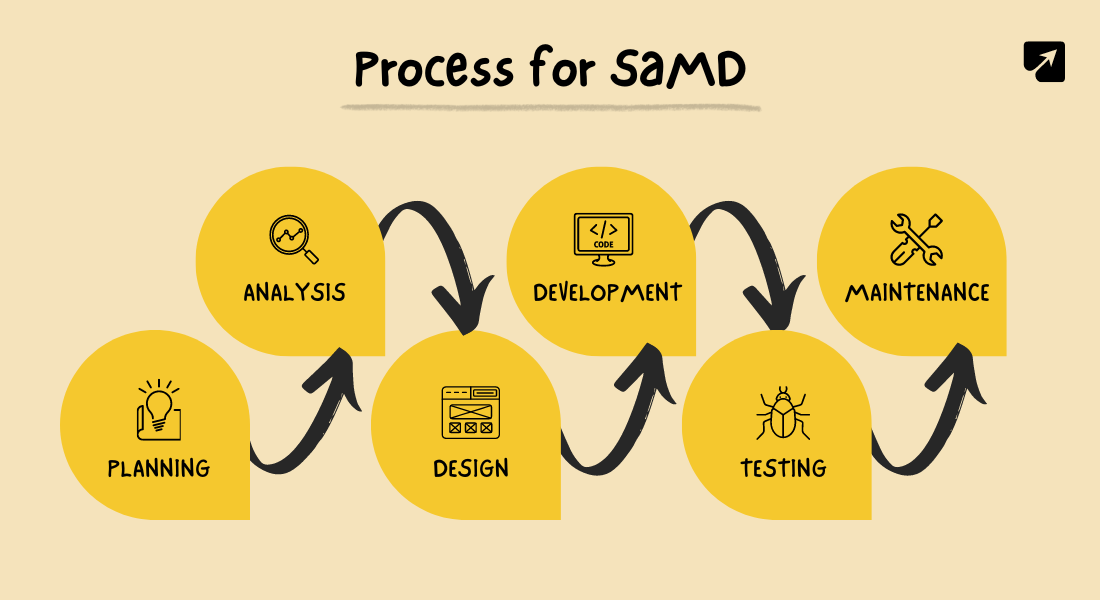
The Software Development Process for Software as a Medical Device (SaMD) involves several key steps to ensure the software is safe, effective, and meets regulatory standards.
Throughout the process, collaboration among developers, healthcare professionals, and regulatory experts is essential to create a SaMD that truly benefits patients and healthcare providers.
To sum up, the growth of Software as a Medical Device (SaMD) is a turning point in the healthcare industry, introducing new possibilities for development, patient consideration, and clinical management. As the lines between technology and healthcare keep on obscuring, the capability of a medical services application improvement business turns out to be progressively important.
These firms are at the forefront of creating and carrying out software solutions that work on clinical results, yet additionally smooth out medical services cycles and increment patient contribution. The change achieved by SaMD shows the groundbreaking force of digital health technologies, offering a future in which medical services are more open, effective, and customized.
As we move forward, collaborating with a skilled Healthcare App Development Company will be critical for any organization seeking to fully realize the potential of SaMD and promote genuine change in the healthcare industry.
Q1: Do patients use SaMD at home or in hospitals and clinics?
Yes, Patients can use SaMD both at home and in hospitals and clinics.
Q2: Does SaMD always need an internet connection?
No, SaMD does not always need an internet connection to function.
Q3: What does SaMD stand for?
SaMD stands for Software as a Medical Device
Q4: What is the role of software as a medical device?
The role of SaMD is to diagnose, treat, or manage health conditions without being part of traditional hardware.
Q5: Is SaMD a medical device?
Yes, SaMD is considered a medical device by regulatory authorities.